Abstract
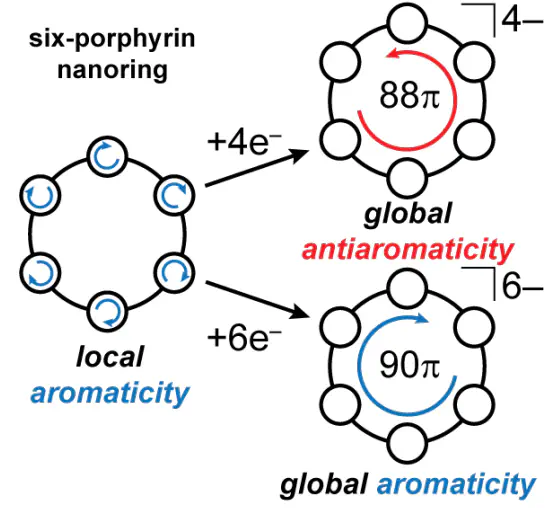
Doping, through oxidation or reduction, is often used to modify the properties of π-conjugated oligomers. In most cases, the resulting charge distribution is difficult to determine. If the oligomer is cyclic and doping establishes global aromaticity or antiaromaticity, then it is certain that the charge is fully delocalized over the entire perimeter of the ring. Herein we show that reduction of a six-porphyrin nanoring using decamethylcobaltocene results in global aromaticity (in the 6− state; [90 π]) and antiaromaticity (in the 4− state; [88 π]), consistent with the Hückel rules. Aromaticity is assigned by NMR spectroscopy and density-functional theory calculations.
Type
Publication
Angewandte Chemie International Edition