Photoexcited and ground-state diradical(oid) character in a triquino[3]radialene
Abstract
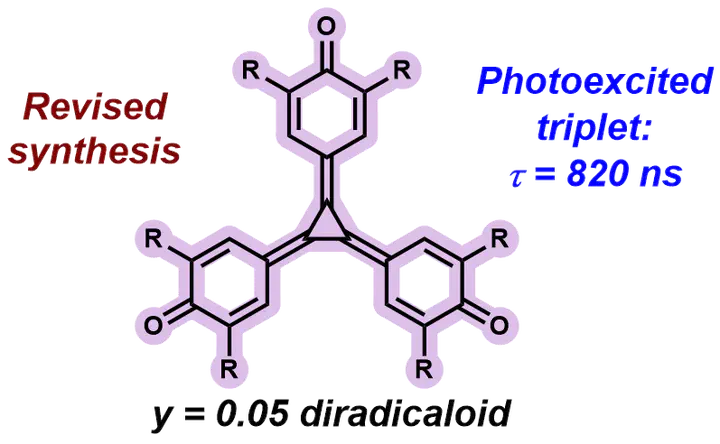
The unusual [3]radialene motif at the centre of triquino[3]radialene permits exceptional electronic conjugation between the three quinone arms of the molecule, giving rise to a remarkably low-energy and intense absorption spectrum. Since triquino[3]radialene’s initial synthesis in the 1970s, questions have been posed around the molecule’s potential diradical(oid) character. In this work we present new synthetic approaches to triquino[3]radialene and a thorough spectroscopic and computational evaluation of its properties. Despite broad UV-visible absorption, the triquino[3]radialene is non-emissive. An investigation by transient absorption spectroscopy reveals that the initially-formed excited state of triquino[3]radialene decays to a triplet state with a lifetime of 0.82 µs. Using computational chemistry, we compare triquino[3]radialene to Yang’s biradical and galvionxyl, with which it is structurally similar. DFT reveals insights into the role of aromaticity in the photoexcited triplet state, where quinoidal rings become more aromatic. MCSCF and CIPT2 calculations reproduce the experimental spectra and reveal a diradical character of y=0.05 in triquino[3]radialene.
Add the full text or supplementary notes for the publication here using Markdown formatting.